Helps reduce fibromyalgia symptoms
Quell Fibromyalgia helps reduce lower extremity pain and other fibromyalgia symptoms in patients with high-pain sensitivity.
Non-invasive and drug-free
Quell Fibromyalgia has none of the side effects of pharmacological treatments.
Easy and convenient
Quell Fibromyalgia is easy for your patients to setup and use at home. Your staff time will not be required. If your patients have questions, they can speak with our Boston based customer care team.
Advanced wearable technology
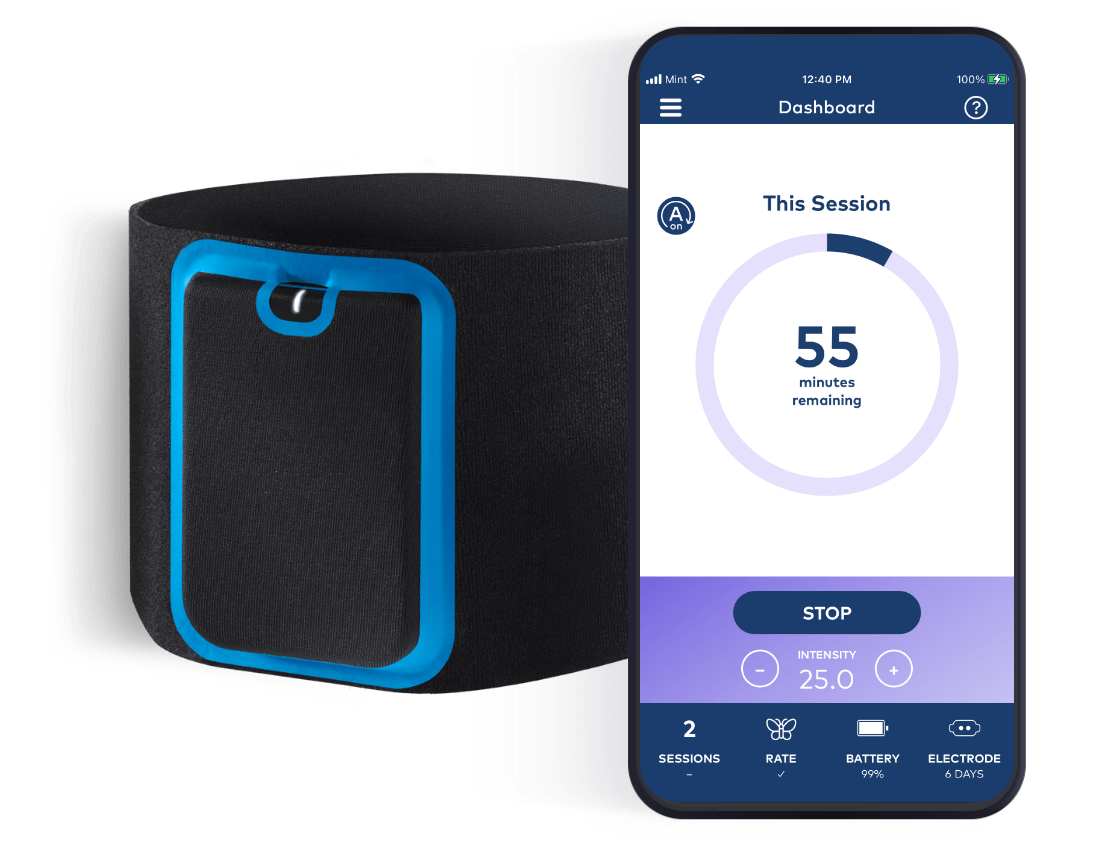
Quell Fibromyalgia seamlessly integrates with a mobile app to create a digital health experience.
First and only FDA authorized medical device to help reduce fibromyalgia symptoms
Quell Fibromyalgia is a Class II medical device. It received FDA De Novo authorization (DEN210046) in May 2022. The device is indicated as an aid for reducing the symptoms of fibromyalgia in adults with high pain sensitivity. The device may be used during sleep.
The product labeling should be reviewed for a complete list of limitations, contraindications, precautions and warnings.
View Prescribing Information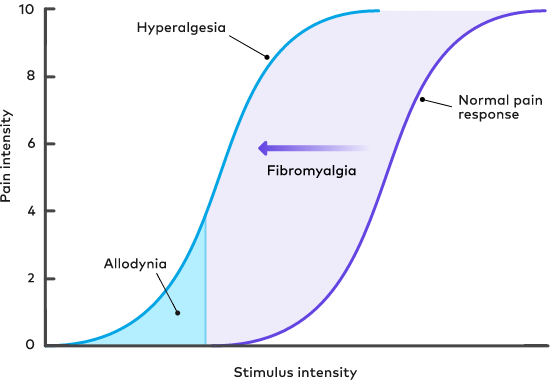
For fibromyalgia patients with high pain sensitivity
The spectrum of pain sensitivity in fibromyalgia patients ranges from normal sensitivity comparable to healthy controls to severe disabling sensitivity. Patients with high pain sensitivity report hyperalgesia and allodynia, such as excessive tenderness to touch, painful reactions to cold and/or heat, and pain in response to light touch. Patients with fibromyalgia and high pain sensitivity have a lower quality of life and greater disease impact than those with normal pain sensitivity.
Quell Fibromyalgia was studied in a double-blind, randomized, sham-controlled trial1

- 119 subjects with physician diagnosed fibromyalgia who also met the American College of Rheumatology 2010 diagnostic criteria.
- Subjects randomized to active or sham treatment for 3-months.
- Analyses conducted on the intention-to-treat population (ITT, N=119) and a prespecified high pain sensitivity subgroup (N=60). The primary endpoint was a statistically significant mean difference in the 3-month PGIC2 scores between the treatment arms.
Key Study Results (in high pain sensitivity subgroup3)
58% of subjects in the active arm compared to 30% in the sham arm (p=0.025) reported at least moderately better symptoms and overall quality of life.2
58% of subjects in the active arm compared to 28% in the sham arm (p=0.019) reported a clinically meaningful reduction (≥15%) in fibromyalgia impact.4
60% of subjects in the active arm compared to 18% in the sham arm (p<0.001) reported at least a moderate reduction (≥30%) in pain intensity.5,6
Note: The percentage of patients expected to obtain meaningful symptom improvement in routine clinical practice has not yet been determined.
Mechanisms of action
The specific biological mechanisms by which Quell Fibromyalgia reduces lower extremity pain and other symptoms of fibromyalgia are uncertain. However, several neurological processes may be relevant.

- The biological trigger for pain relief from transcutaneous electrical nerve stimulation is believed to be persistent peripheral nerve stimulation at an intensity above the sensory threshold.7
- The original model for how sensory nerve stimulation produces pain relief was proposed by Melzack and Wall in 1965. Their theory stipulated that activation of large diameter sensory nerves (Aβ fibers) closes a “pain gate” in the spinal cord that inhibits the onward transmission of pain signals carried by nociceptive afferents (C and Aδ) to the brain.8
- An expansion of the pain gate theory attributes the clinical benefits of transcutaneous electrical nerve stimulation to a reduction in central excitation and an enhancement of central inhibition. This model is aligned to the understanding of fibromyalgia as condition of central sensitization, which is an overamplification of pain and other sensory signals in the central nervous system.9
Prescribing Quell Fibromyalgia is straightforward
The decision to prescribe Quell Fibromyalgia is made between you and your patient. There is no need to collect burdensome documentation and then wait for an insurance decision.
To learn more about diagnosing fibromyalgia, view our Fibromyalgia Diagnostic Criteria worksheet.

Review indications for use and contraindications
Quell Fibromyalgia is indicated as an aid for reducing the symptoms of fibromyalgia in adults with high pain sensitivity. The device may be used during sleep.
The device is contraindicated for use by patients who have a cardiac pacemaker, implanted defibrillator, other implanted electronic device, or implanted metal near the device.


Submit prescription
Quell Fibromyalgia prescriptions are processed by fax through HealthWarehouse.com, a national online pharmacy. You may also call them by phone.


Pharmacy contacts your patient
Upon receiving the prescription, the pharmacy will contact your patient (typically within 1 business day) to collect required health information and payment details. To speed up the process, your patient can go to healthwarehouse.ac-page.com/quell-update to order their device and provide the required information. Quell Fibromyalgia will then be shipped for arrival within 2-3 days. Your patients will be guided step-by-step through the device setup process by the mobile app. If they have any questions, they can speak with our Boston based customer service team.